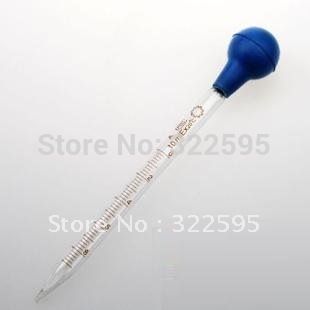
The Physics of Pipettes:
So I
was in lab the other day and, of course, we were doing a lot of pipetting (its chemistry, after all) which made me
think about how a pipette actually works. What I saw is that when you squeeze
the pipette bulb, air is ejected from the pipette. This is because you are
decreasing the volume of the pipette and, since it is an open container, air
leaves so that the pressure inside is the same as that outside. When you
release the bulb, the volume increases. But since the tip is now in the liquid,
the pipette is now a closed container (gas from the atmosphere can’t get in). Therefore
when the volume increases, the gas inside expands to fill the container and so
the pressure decreases. With less pressure inside the pipette, there is less
force pressing down on the liquid inside than there is outside, so the liquids
is drawn into the pipette so that the pressure inside equalizes with that
outside (Pascal’s Principle). I decided to find out how much you must decrease
the pressure inside the pipette to draw up 5 mL of water. For this case, we are
using a 10 mL graduated pipette and assuming that the volume of the pipette
bulb is 5 mL as well.
To do
this I applied Boyle’s Law, which states that the initial pressure times the
initial volume is equal to the final pressure times the final volume. Our
initial state is when the bulb of the pipette is compressed and thus the
pressure is equal to the atmospheric pressure. The final state is the instant when the bulb
has been released but no water yet drawn up. We also must make some
assumptions. These are that the total volume of the pipette is 20 mL and that
the volume of the uncompressed bulb is 5 mL.
P1V1=P2V2
P
1=atmosphere (P
A)
V1=V2-5mL
PA(V2-5mL) =P2V2
(1.013e5 Pa)(25mL-5mL)=P2(25mL)
P2=8.104x104 Pa
∆P=PA-P2=1.013e5
Pa – 8.104x104 Pa
∆P=2.026x104
Pa

Thanks for sharing the such a informative thingsMicropipettes calibration lab
ReplyDelete